Neuroimaging Techniques for Neuroplasticity Quantification in Stroke Patients
DOI:
https://doi.org/10.17488/RMIB.44.2.5Keywords:
diffusion tensor imaging, functional magnetic resonance imaging, neuroimaging, neuroplasticity, strokeAbstract
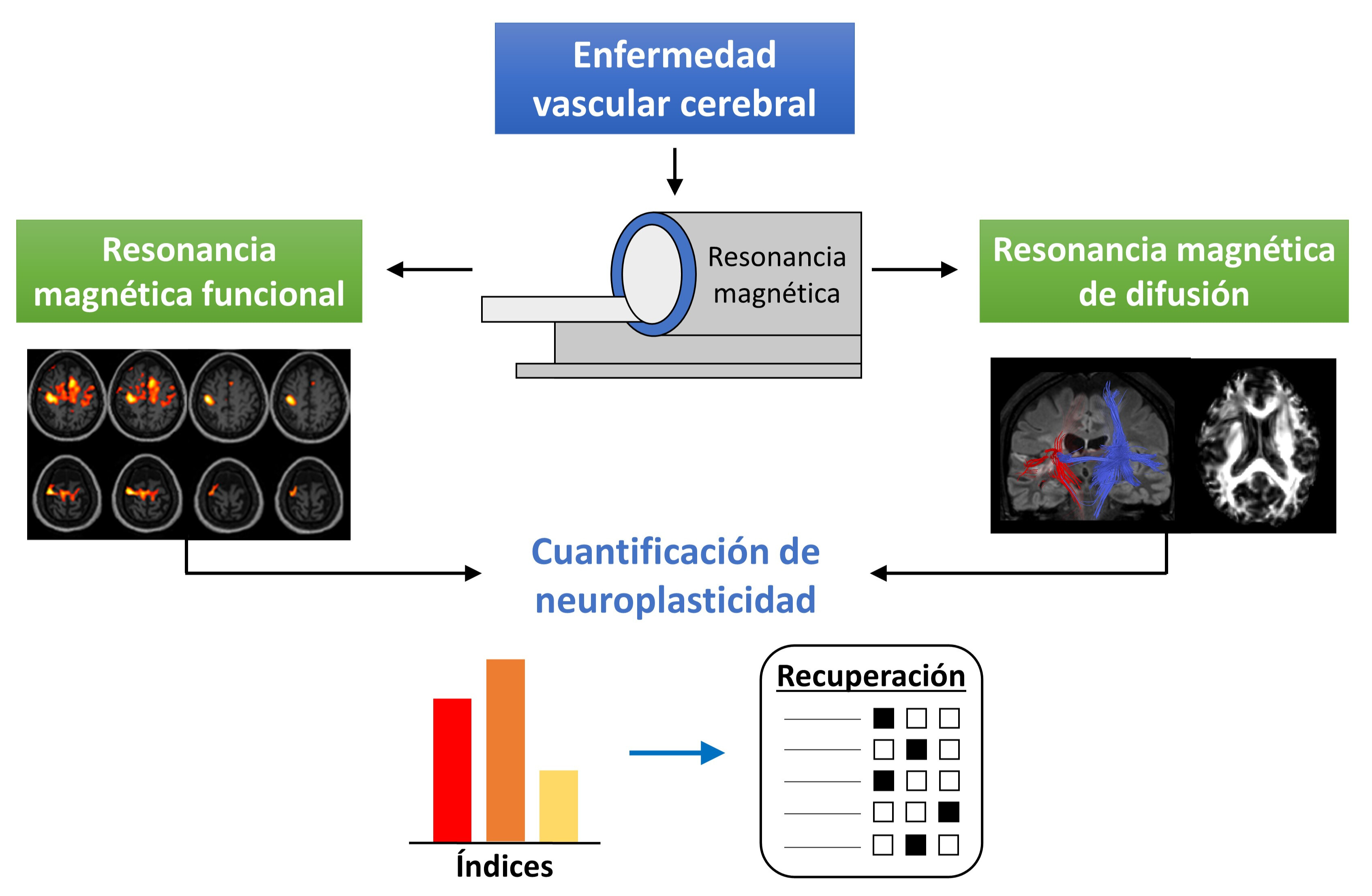
Neuroimaging techniques provide relevant information of the functional and anatomical status of the human brain. This information is of particular importance when a pathology, like stroke, produces a brain injury. In stroke patients, it has been determined that neuroplasticity is the primary recovery mechanism of the lost motor function. Due to worldwide high prevalence, especially in developing countries, it is necessary to continue the research of the recovery mechanisms involved in this pathology. To this end, functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) are two of the most used neuroimaging techniques. In stroke patients, fMRI allows the analysis of the neural activity produced by the execution of motor tasks, whereas DTI provides structural information of the brain anatomy. In this narrative review, multiple studies that employ these neuroimaging techniques for quantification of neuroplasticity changes in stroke patients after undergoing a neurorehabilitation program are presented. Better understanding of these neuroplasticity changes would allow researchers to design and provide more beneficial rehabilitation schemes to stroke patients.
Downloads
References
V. L. Feigin, B. A. Stark, C. O. Johnson, G. A. Roth, C. Bisignano, et al., “Global, regional, and national burden of stroke and its risk factors, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019,” Lancet Neurol., vol. 20, no. 10, pp. 795-820, Oct. 2021, doi: https://doi.org/10.1016/S1474-4422(21)00252-0
V. De la Cruz-Góngora, E. Chiquete, H. Gómez–Dantés, L. Cahuana-Hurtado, C. Cantú-Brito, “Trends in the burden of stroke in Mexico: A national and subnational analysis of the global burden of disease 1990–2019,” Lancet Reg. Health Am., vol. 10, art. no. 100204, Jun. 2022, doi: https://doi.org/10.1016/j.lana.2022.100204
C. Grefkes, G. R. Fink, “Recovery from stroke: current concepts and future perspectives.,” Neurol. Res. Pract., vol. 2, art. no. 17, Jun. 2020, doi: https://doi.org/10.1186/s42466-020-00060-6
B. H. Dobkin, “Clinical practice. Rehabilitation after stroke.,” N. Engl. J. Med., vol. 352, pp. 1677–1684, Apr. 2005, doi: https://doi.org/10.1056/NEJMcp043511
L. M. Carey, D. F. Abbott, G. F. Egan, et al., “Evolution of Brain Activation with Good and Poor Motor Recovery after Stroke,” Neurorehabil. Neural Repair, vol. 20, no. 1, pp. 24–41, Mar. 2006, doi: https://doi.org/10.1177/1545968305283053
R. Mane, T. Chouhan, C. Guan, “BCI for stroke rehabilitation: motor and beyond.,” J. Neural Eng., vol. 17, no. 4, art. no. 41001, Aug. 2020, doi: https://doi.org/10.1088/1741-2552/aba162
K. C. Dodd, V. A. Nair, and V. Prabhakaran, “Role of the Contralesional vs. Ipsilesional Hemisphere in Stroke Recovery,” Front. Hum. Neurosci., vol. 11, art. no. 469, Sep. 2017, doi: https://doi.org/10.3389/fnhum.2017.00469
L. Carey, A. Walsh, A. Adikari, P. Godin, et al., “Finding the Intersection of Neuroplasticity, Stroke Recovery, and Learning: Scope and Contributions to Stroke Rehabilitation.,” Neural Plast., vol. 2019, art. no. 5232374, May. 2019, doi: https://doi.org/10.1155/2019/5232374
S. C. Cramer, M. Sur, B. H. Dobkin, C. O'Brien, et al., “Harnessing neuroplasticity for clinical applications.,” Brain, vol. 134, no. 6, pp. 1591–1609, Jun. 2011, doi: https://doi.org/10.1093/brain/awr039
W. T. Greenough, J. E. Black, C. S. Wallace, “Experience and Brain Development,” Child Dev., vol. 58, No. 3, pp. 539–559, Jun. 1987, doi: https://doi.org/10.2307/1130197
Y. Chang, “Reorganization and plastic changes of the human brain associated with skill learning and expertise,” Front. Hum. Neurosci., vol. 8, art. no. 35, 2014, doi: https://doi.org/10.3389/fnhum.2014.00035
L. G. Ungerleider, J. Doyon, A. Karni, “Imaging Brain Plasticity during Motor Skill Learning,” Neurobiol. Learn. Mem., vol. 78, no. 3, pp. 553–564, Nov. 2002, doi: https://doi.org/10.1006/nlme.2002.4091
A. May, “Experience-dependent structural plasticity in the adult human brain,” Trends Cogn. Sci., vol. 15, no. 10, pp. 475–482, Oct. 2011, doi: https://doi.org/10.1016/j.tics.2011.08.002
N. Dancause S. Barbay, S. B. Frost, “Extensive Cortical Rewiring after Brain Injury,” J. Neurosci., vol. 25, no. 44, pp. 10167–10179, Nov. 2005, doi: https://doi.org/10.1523/JNEUROSCI.3256-05.2005
T. Murphy, D. Corbett, “Plasticity during stroke recovery: from synapse to behaviour,” Nat. Rev. Neurosci., vol. 10, pp. 861–872, Dec. 2009, doi: https://doi.org/10.1038/nrn2735
M. Pekna, M. Pekny, M. Nilsson, “Modulation of Neural Plasticity as a Basis for Stroke Rehabilitation,” Stroke, vol. 43, no. 10, pp. 2819–2828, Oct. 2012, doi: https://doi.org/10.1161/STROKEAHA.112.654228
T. V Bliss, T. Lømo, “Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path,” J. Physiol., vol. 232, no. 2, pp. 331–356, Jul. 1973, doi: https://doi.org/10.1113/jphysiol.1973.sp010273
R. A. Nicoll, “A Brief History of Long-Term Potentiation.,” Neuron, vol. 93, no. 2, pp. 281–290, Jan. 2017, doi: https://doi.org/10.1016/j.neuron.2016.12.015
S. Peineau, C. Taghibiglou, C. Bradley, T. P. Wong, L. Liu, J. Lu, et al., “LTP inhibits LTD in the Hippocampus via Regulation of GSK3beta,” Neuron, vol. 53, no. 5, pp. 703–717, Mar. 2007, doi: https://doi.org/10.1016/j.neuron.2007.01.029
A. Mancini, A. de Iure, B. Picconi, “Basic mechanisms of plasticity and learning,” in Handbook of Clinical Neurology, A. Quartarone, M. F. Ghilardi, F. Boller, Eds., Straive, India: Elsevier, 2022, ch. 2, pp. 21–34, doi: https://doi.org/10.1016/B978-0-12-819410-2.00002-3
S. C. Cramer, M. Chopp, “Recovery recapitulates ontogeny,” Trends Neurosci., vol. 23, no. 6, pp. 265–271, Jun. 2000, doi: https://doi.org/10.1016/s0166-2236(00)01562-9
T. Wieloch, K. Nikolich, “Mechanisms of neural plasticity following brain injury,” Curr. Opin. Neurobiol., vol. 16, no. 3, pp. 258–264, Jun. 2006, doi: https://doi.org/10.1016/j.conb.2006.05.011
S. T. Carmichael, “Emergent properties of neural repair: elemental biology to therapeutic concepts,” Ann. Neurol., vol. 79, no. 6, pp. 895–906, Jun. 2016, doi: https://doi.org/10.1002/ana.24653
A. Durukan, T. Tatlisumak, “Acute ischemic stroke: Overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia,” Pharmacol. Biochem. Behav., vol. 87, no. 1, pp. 179–197, May 2007, doi: https://doi.org/10.1016/j.pbb.2007.04.015
L. A. Boyd, K. S. Hayward, N. S. Ward, et al., “Biomarkers of stroke recovery: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable,” Int. J. Stroke, vol. 12, no. 5, pp. 480–493, Jul. 2017, doi: https://doi.org/10.1177/1747493017714176
C. Grefkes, G. R. Fink, “Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches,” Brain, vol. 134, no. 5, pp. 1264–1276, May 2011, doi: https://doi.org/10.1093/brain/awr033
S. Ogawa, D. W. Tank, R. Menor, J. M. Ellermann, et al., “Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging,” Proc. Natl. Acad. Sci., vol. 89, no. 13, pp. 5951–5955, Jul. 1992, doi: https://doi.org/10.1073/pnas.89.13.5951
D. Attwell, C. Iadecola, “The neural basis of functional brain imaging signals,” Trends Neurosci., vol. 25, no. 12, pp. 621–625, Dec. 2002, doi: https://doi.org/10.1016/s0166-2236(02)02264-6
A. Crofts, M. E. Kelly, C. L. Gibson, “Imaging Functional Recovery Following Ischemic Stroke: Clinical and Preclinical fMRI Studies,” J. Neuroimaging, vol. 30, no. 1, pp. 5–14, Jan. 2020, doi: https://doi.org/10.1111/jon.12668
N. S. Ward, M. M. Brown, A. J. Thompson, R. S. J. Frackowiak, “Neural correlates of motor recovery after stroke: a longitudinal fMRI study,” Brain, vol. 126, no. 11, pp. 2476–2496, Nov. 2003, doi: https://doi.org/10.1093/brain/awg245
D. A. Nowak, C. Grefkes, M. Dafotakis, S. Eickhoff, et al., “Effects of Low-Frequency Repetitive Transcranial Magnetic Stimulation of the Contralesional Primary Motor Cortex on Movement Kinematics and Neural Activity in Subcortical Stroke,” Arch. Neurol., vol. 65, no. 6, pp. 741–747, Jun. 2008, doi: https://doi.org/10.1001/archneur.65.6.741
A. K. Rehme, S. B. Eickhoff, C. Rottschy, G. R. Fink, C. Grefkes, “Activation likelihood estimation meta-analysis of motor-related neural activity after stroke,” Neuroimage, vol. 59, no. 3, pp. 2771–2782, Feb. 2012, doi: https://doi.org/10.1016/j.neuroimage.2011.10.023
A. K. Rehme, S. B. Eickhoff, L. E. Wang, G. R. Fink, C. Grefkes, “Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke,” Neuroimage, vol. 55, no. 3, pp. 1147–1158, Apr. 2011, doi: https://doi.org/10.1016/j.neuroimage.2011.01.014
A. K. Rehme, G. R. Fink, D. Y. von Cramon, C. Grefkes, “The Role of the Contralesional Motor Cortex for Motor Recovery in the Early Days after Stroke Assessed with Longitudinal fMRI,” Cereb. Cortex, vol. 21, no. 4, pp. 756–768, Apr. 2011, doi: https://doi.org/10.1093/cercor/bhq140
L. E. Wang, G. R. Fink, S. Diekhoff, A. K. Rehme, S. B. Eickhoff, C. Grefkes, “Noradrenergic enhancement improves motor network connectivity in stroke patients,” Ann. Neurol., vol. 69, no. 2, pp. 375–388, Feb. 2011, doi: https://doi.org/10.1002/ana.22237
C. Grefkes, G. R. Fink, “Connectivity-based approaches in stroke and recovery of function,” Lancet Neurol., vol. 13, no. 2, pp. 206–216, Feb. 2014, doi: https://doi.org/10.1016/S1474-4422(13)70264-3
S. Mori, J. Zhang, “Principles of Diffusion Tensor Imaging and Its Applications to Basic Neuroscience Research,” Neuron, vol. 51, no. 5, pp. 527–539, Sep. 2006, doi: https://doi.org/10.1016/j.neuron.2006.08.012
Y. J. Chen, S. A. Nabavizadeh, A. Vossough, S. Kumar, L. A. Loevner, S. Mohan, “Wallerian Degeneration Beyond the Corticospinal Tracts: Conventional and Advanced MRI Findings,” J. Neuroimaging, vol. 27, no. 3, pp. 272–280, May 2017, doi: https://doi.org/10.1111/jon.12404
J. Puig, G. Blasco, J. Daunis-I-Estadella, G. Thomalla, M. Castellanos, et al., “Decreased Corticospinal Tract Fractional Anisotropy Predicts Long-term Motor Outcome After Stroke,” Stroke, vol. 44, no. 7, pp. 2016–2018, Jul. 2013, doi: https://doi.org/10.1161/STROKEAHA.111.000382
J. Song, V. A. Nair, B. M. Young, L. M. Walton, et al., “DTI measures track and predict motor function outcomes in stroke rehabilitation utilizing BCI technology,” Front. Hum. Neurosci., vol. 9, art. no. 195, Apr. 2015, doi: https://doi.org/10.3389/fnhum.2015.00195
E. V. R. DiBella, A. Sharma, L. Richards, V. Prabhakaran, J. J. Majersik, and S. K. HashemizadehKolowri, “Beyond Diffusion Tensor MRI Methods for Improved Characterization of the Brain after Ischemic Stroke: A Review,” AJNR Am. J. Neuroradiol., vol. 43, no. 5, pp. 661–669, May 2022, doi: https://doi.org/10.3174/ajnr.A7414
W. Feng, J. Wang, P. Y. Chhatbar, C. Doughty, D. Landsittel, et al., “Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes,” Ann. Neurol., vol. 78, no. 6, pp. 860–870, Dec. 2015, doi: https://doi.org/10.1002/ana.24510
P. Kumar, A. K. Yadav, S. Misra, A. Kumar, K. Chakravarty, K. Prasad, “Prediction of upper extremity motor recovery after subacute intracerebral hemorrhage through diffusion tensor imaging: a systematic review and meta-analysis,” Neuroradiology, vol. 58, no. 10, pp. 1043–1050, Oct. 2016, doi: https://doi.org/10.1007/s00234-016-1718-6
P. Kumar, P. Kathuria, P. Nair, K. Prasad, “Prediction of Upper Limb Motor Recovery after Subacute Ischemic Stroke Using Diffusion Tensor Imaging: A Systematic Review and Meta-Analysis,” J. Stroke, vol. 18, no. 1, pp. 50–59, Jan. 2016, doi: https://doi.org/10.5853/jos.2015.01186
R. T. Pivik, R. J. Broughton, R. Coppola, R. J. Davidson, N. Fox, M. R. Nuwer, “Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts,” Psychophysiology, vol. 30, no. 6, pp. 547–558, Nov. 1993, doi: https://doi.org/10.1111/j.1469-8986.1993.tb02081.x
M. L. Seghier, “Laterality index in functional MRI: methodological issues,” Magn. Reson. Imaging, vol. 26, no. 5, pp. 594–601, Jun. 2008, doi: https://doi.org/10.1016/j.mri.2007.10.010
J. E. Desmond, J. M. Sum, A. D. Wagner, J. B. Demb et al., “Functional MRI measurement of language lateralization in Wada-tested patients,” Brain, vol. 118, no. 6, pp. 1411–1419, Dec. 1995, doi: https://doi.org/10.1093/brain/118.6.1411
J. R. Binder, S. J. Swanson, T. A. Hammeke, G. L. Morris, et al., “Determination of language dominance using functional MRI: a comparison with the Wada test,” Neurology, vol. 46, no. 4, pp. 978–984, Apr. 1996, doi: https://doi.org/10.1212/wnl.46.4.978
C. Doughty, J. Wang, W. Feng, D. Hackney, E. Pani, G. Schlaug, “Detection and Predictive Value of Fractional Anisotropy Changes of the Corticospinal Tract in the Acute Phase of a Stroke,” Stroke, vol. 47, no. 6, pp. 1520–1526, Jun. 2016, doi: https://doi.org/10.1161/STROKEAHA.115.012088
J. Griauzde, A. Srinivasan, “Advanced Neuroimaging Techniques: Basic Principles and Clinical Applications,” J. Neuroophthalmol., vol. 38, no. 1, pp. 101–114, Mar. 2018, doi: https://doi.org/10.1097/WNO.0000000000000539
R. A. Poldrack, J. A. Mumford, T. E. Nichols, Handbook of Functional MRI Data Analysis. Cambridge, UK: Cambridge University Press, 2011, pp. 70.
S. Saini, R. B. Frankel, D. D. Stark, J. T. Ferrucci, “Magnetism: a primer and review,” AJR Am. J. Roentgenol., vol. 150, no. 4, pp. 735–743, Apr. 1988, doi: https://doi.org/10.2214/ajr.150.4.735
S. Ogawa, T.-M. Lee, A. S. Nayak, P. Glynn, “Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields,” Magn. Reson. Med., vol. 14, no. 1 pp. 68–78, Apr. 1990, doi: https://doi.org/10.1002/mrm.1910140108
J. M. Soares, R. Magalhães, P. S. Moreira, A. Sousa, et al., “A Hitchhiker’s Guide to Functional Magnetic Resonance Imaging,” Front. Neurosci., vol. 10, art. no. 515 Nov. 2016, doi: https://doi.org/10.3389/fnins.2016.00515
L. Hay, A. H. B. Duffy, S. J. Gilbert, M. A. Grealy, “Functional magnetic resonance imaging (fMRI) in design studies: Methodological considerations, challenges, and recommendations,” Des. Stud., vol. 78, art. no. 101078, Jan. 2022, doi: https://doi.org/10.1016/j.destud.2021.101078
S.-G. Kim, E. Rostrup, H. B. W. Larsson, S. Ogawa, O. B. Paulson, “Determination of relative CMRO2 from CBF and BOLD changes: Significant increase of oxygen consumption rate during visual stimulation,” Magn. Reson. Med., vol. 41, no. 6, pp. 1152–1161, Jun. 1999, doi: https://doi.org/10.1002/(SICI)1522-2594(199906)41:6<1152::AID-MRM11>3.0.CO;2-T
C. M. Bennett, M. B. Miller, “fMRI reliability: Influences of task and experimental design,” Cogn. Affect. Behav. Neurosci., vol. 13, no. 4, pp. 690–702, 2013, doi: https://doi.org/10.3758/s13415-013-0195-1
A. Caria, C. Weber, D. Brötz, A. Ramos, et al., “Chronic stroke recovery after combined BCI training and physiotherapy: A case report,” Psychophysiology, vol. 48, no. 4 pp. 578–582, Apr. 2011, doi: https://doi.org/10.1111/j.1469-8986.2010.01117.x
B. M. Young, Z. Nigogosyan, L. M. Walton, A. Remsik, et al., “Dose-response relationships using brain-computer interface technology impact stroke rehabilitation,” Front. Hum. Neurosci., vol. 9, art. no. 361, Jun. 2015, doi: https://doi.org/10.3389/fnhum.2015.00361
A. Ramos-Murguialday, M. R. Curado, D. Broetz, Ö. Yilmaz, et al., “Brain-Machine Interface in Chronic Stroke: Randomized Trial Long-Term Follow-up,” Neurorehabil. Neural Repair, vol. 33, no. 3, pp. 188–198, Mar. 2019, doi: https://doi.org/10.1177/1545968319827573
K. Yuan, X. Wang, C. Chen, C. C.-Y. Lau, et al., “Interhemispheric Functional Reorganization and its Structural Base After BCI-Guided Upper-Limb Training in Chronic Stroke,” IEEE Trans. Neural Syst. Rehabilitation Eng., vol. 28, no. 11, pp. 2525–2536, Nov. 2020, doi: https://doi.org/10.1109/TNSRE.2020.3027955
M. Demers, R. Varghese, C. Winstein, “Retrospective Analysis of Task-Specific Effects on Brain Activity After Stroke: A Pilot Study,” Front. Hum. Neurosci., vol. 16, art. no. 871239, 2022, doi: https://doi.org/10.3389/fnhum.2022.871239
S. E. Petersen, J. W. Dubis, “The mixed block/event-related design,” Neuroimage, vol. 62, no. 2, pp. 1177–1184, Aug. 2012, doi: https://doi.org/10.1016/j.neuroimage.2011.09.084
M. Welvaert, Y. Rosseel, “A Review of fMRI Simulation Studies,” PLoS One, vol. 9, no. 7, art. no. e101953, Jul. 2014, doi: https://doi.org/10.1371/journal.pone.0101953
J. Saunders, H. L. Carlson, F. Cortese, B. G. Goodyear, A. Kirton, “Imaging functional motor connectivity in hemiparetic children with perinatal stroke,” Hum. Brain Mapp., vol. 40, no. 5, pp. 1632–1642, Apr. 2019, doi: https://doi.org/10.1002/hbm.24474
G. Lioi, S. Bulet, M. Fleury, E. Bannier, et al., “A Multi-Target Motor Imagery Training Using Bimodal EEG-fMRI Neurofeedback: A Pilot Study in Chronic Stroke Patients,” Front. Hum. Neurosci., vol. 14, art. no. 37, Feb. 2020, doi: https://doi.org/10.3389/fnhum.2020.00037
S.-L. Liew, K. A. Garrison, K. L. Ito, P. Heydari, et al., “Laterality of Poststroke Cortical Motor Activity during Action Observation Is Related to Hemispheric Dominance,” Neural Plast., vol. 2018, p. 3524960, May. 2018, doi: https://doi.org/10.1155/2018/3524960
A. Errante, D. Saviola, M. Cantoni, K. Iannuzzelli, et al., “Effectiveness of action observation therapy based on virtual reality technology in the motor rehabilitation of paretic stroke patients: a randomized clinical trial,” BMC Neurol., vol. 22, no. 1, art. no. 109, Mar. 2022, doi: https://doi.org/10.1186/s12883-022-02640-2
J. B. Kroth, B. Handfas, G. Rodrigues, F. Zepeda, et al., “Effects of Repetitive Peripheral Sensory Stimulation in the Subacute and Chronic Phases After Stroke: Study Protocol for a Pilot Randomized Trial,” Front. Neurol., vol. 13, art. no. 779128, Feb. 2022, doi: https://doi.org/10.3389/fneur.2022.779128
A. Lasek-Bal, J. Kidoń, M. Błaszczyszyn, B. Stasiów, A. Żak, “BOLD fMRI signal in stroke patients and its importance for prognosis in the subacute disease period - Preliminary report,” Neurol. Neurochir. Pol., vol. 52, no. 3, pp. 341–346, May. 2018, doi: https://doi.org/10.1016/j.pjnns.2017.12.006
W. Penny, K. Friston, J. Ashburner, S. Kiebel, T. Nichols, Eds., Statistical Parametric Mapping: The Analysis of Functional Brain Images. London, UK: Academic Press, 2007 pp. 5.
J. Ashburner, G. Barnes, C.-C. Chen, G. Flandin, et al. SPM12 Manual. (2021). Accessed: Jun. 10, 2023. [Online]. Available: https://www.fil.ion.ucl.ac.uk/spm/doc/spm12_manual.pdf
A. Caria, J. L. D. da Rocha, G. Gallitto, N. Birbaumer, R. Sitaram, A. R. Murguialday, “Brain-Machine Interface Induced Morpho-Functional Remodeling of the Neural Motor System in Severe Chronic Stroke,” Neurotherapeutics, vol. 17, no. 2, pp. 635–650, Apr. 2020, doi: https://doi.org/10.1007/s13311-019-00816-2
A. Ramos-Murguialday, D. Broetz, M. Rea, L. Läer, et al., “Brain-machine interface in chronic stroke rehabilitation: A controlled study,” Ann. Neurol., vol. 74, no. 1, pp. 100–108, Jul. 2013, doi: https://doi.org/10.1002/ana.23879
A. Jansen, R. Menke, J. Sommer, A. F. Förster, et al., “The assessment of hemispheric lateralization in functional MRI—Robustness and reproducibility,” Neuroimage, vol. 33, no. 1, pp. 204–217, Oct. 2006, doi: https://doi.org/10.1016/j.neuroimage.2006.06.019
E. T. Rolls, C.-C. Huang, C.-P. Lin, J. Feng, M. Joliot, “Automated anatomical labelling atlas 3,” Neuroimage, vol. 206, art. no. 116189, Feb. 2020, doi: https://doi.org/10.1016/j.neuroimage.2019.116189
M. Wilke, K. Lidzba, “LI-tool: A new toolbox to assess lateralization in functional MR-data,” J. Neurosci. Methods, vol. 163, no. 1, pp. 128–136, Jun. 2007, doi: https://doi.org/10.1016/j.jneumeth.2007.01.026
MATLAB 9.11.0. (2001). The MathWorks, Inc. Accessed: Jun. 10, 2023. [Online]. Available: https://www.mathworks.com
S. Vulliemoz, O. Raineteau, D. Jabaudon, “Reaching beyond the midline: why are human brains cross wired?,” Lancet Neurol., vol. 4, no. 2, pp. 87–99, Feb. 2005, doi: https://doi.org/10.1016/S1474-4422(05)00990-7
C. M. Stinear, P. A. Barber, P. R. Smale, J. P. Coxon, M. K. Fleming, W. D. Byblow, “Functional potential in chronic stroke patients depends on corticospinal tract integrity,” Brain, vol. 130, no. 1, pp. 170–180, Jan. 2007, doi: https://doi.org/10.1093/brain/awl333
G. Schlaug, S. Marchina, C. Y. Wan, “The Use of Non-invasive Brain Stimulation Techniques to Facilitate Recovery from Post-stroke Aphasia,” Neuropsychol. Rev., vol. 21, no. 3, pp. 288–301, Sep. 2011, doi: https://doi.org/10.1007/s11065-011-9181-y
G. Di Pino, G. Pellegrino, G. Assenza, F. Capone, et al., “Modulation of brain plasticity in stroke: a novel model for neurorehabilitation,” Nat. Rev. Neurol., vol. 10, no. 10, pp. 597–608, Oct. 2014, doi: https://doi.org/10.1038/nrneurol.2014.162
J. C. Magee, D. Johnston, “A Synaptically Controlled, Associative Signal for Hebbian Plasticity in Hippocampal Neurons,” Science, vol. 275, no. 5297, pp. 209–213, Jan. 1997, doi: https://doi.org/10.1126/science.275.5297.209
G.-Q. Bi, M.-M. Poo, “Synaptic Modifications in Cultured Hippocampal Neurons: Dependence on Spike Timing, Synaptic Strength, and Postsynaptic Cell Type,” J. Neurosci., vol. 18, no. 24, pp. 10464–10472, Dec. 1998, doi: https://doi.org/10.1523/JNEUROSCI.18-24-10464.1998
G. Q. Bi and M. M. Poo, “Synaptic Modification by Correlated Activity: Hebb’s Postulate Revisited,” Annu. Rev. Neurosci., vol. 24, pp. 139–166, 2001, doi: https://doi.org/10.1146/annurev.neuro.24.1.139
V. Di Lazzaro, P. Profice, F. Capone, P. Pasqualetti, et al., “Motor Cortex Plasticity Predicts Recovery in Acute Stroke,” Cereb. Cortex, vol. 20, no. 7, pp. 1523–1528, Jul. 2010, doi: https://doi.org/10.1093/cercor/bhp216
N. J. J. Beauchamp, A. M. Ulug, T. J. Passe, P. C. van Zijl, “MR diffusion imaging in stroke: review and controversies,” Radiographics, vol. 18, no. 5, pp. 1265–1269, Sep. 1998, doi: https://doi.org/10.1148/radiographics.18.5.9747619
B. M. Young, J. M. Stamm, J. Song, A. B. Remsik, et al., “Brain-Computer Interface Training after Stroke Affects Patterns of Brain-Behavior Relationships in Corticospinal Motor Fibers,” Front. Hum. Neurosci., vol. 10, art. no. 457, Sep. 2016, doi: https://doi.org/10.3389/fnhum.2016.00457
A. Bhasin, P. Srivastava, S. S. Kumaran, “Correlation of DTI-Derived Measures to Therapy-Mediated Recovery after Stroke: Preliminary Findings,” Neurol. India, vol. 69, no. 5, pp. 1210–1216, Oct. 2021 [Online]. Available: https://www.neurologyindia.com/text.asp?2021/69/5/1210/329584
L. M. Moura, R. Luccas, J. P. Q. de Paiva, E. Amaro et al., “Diffusion Tensor Imaging Biomarkers to Predict Motor Outcomes in Stroke: A Narrative Review,” Front. Neurol., vol. 10, art. no. 445, May 2019, doi: https://doi.org/10.3389/fneur.2019.00445
M. Descoteaux, C. Poupon, “Diffusion-Weighted MRI”, in Comprehensive Biomedical Physics, vol. 3, D. Belkić, K. Belkić, Eds., Oxford, United Kingdom: Elsevier, 2014, pp. 81–97.
Y. Okamoto, D. Ishii, S. Yamamoto, M. Wakatabi, et al., “Relationship Between Motor Function, DTI, and Neurophysiological Parameters in Patients with Stroke in the Recovery Rehabilitation unit,” J. Stroke Cerebrovasc. Dis., vol. 30, no. 8, art. no. 105889, Aug. 2021, doi: https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.105889
D. Le Bihan, J.-F. Mangin, C. Poupon, C. A. Clark, et al., “Diffusion tensor imaging: Concepts and applications,” J. Magn. Reson. Imaging, vol. 13, no. 4, pp. 534–546, Apr. 2001, doi: https://doi.org/10.1002/jmri.1076
B. J. Macintosh, S. J. Graham, “Magnetic resonance imaging to visualize stroke and characterize stroke recovery: a review,” Front. Neurol., vol. 4, art. no. 60, May 2013, doi: https://doi.org/10.3389/fneur.2013.00060
K. H. Maier-Hein, P. F. Neher, J.-C. Houde, M. A. Côté, et al., “The challenge of mapping the human connectome based on diffusion tractography,” Nat. Commun., vol. 8, no. 1, art. no. 1349, Nov. 2017, doi: https://doi.org/10.1038/s41467-017-01285-x
W. Van Hecke, L. Emsell, and S. Sunaert, Eds. Diffusion Tensor Imaging: A Practical Handbook. New York, U.S.: Springer New York, 2015, pp. 205.
J.-D. Tournier, F. Calamante, A. Connelly, “MRtrix: Diffusion tractography in crossing fiber regions,” Int. J. Imaging Syst. Technol., vol. 22, pp. 53-66, Feb. 2012, doi: https://doi.org/10.1002/ima.22005
D. Wassermann, N. Makris, Y. Rathi, M. Shenton, R. Kikinis, M. Kubicki, C. F. Westin, “The white matter query language: a novel approach for describing human white matter anatomy,” Brain Struct. Funct., vol. 221, no. 9, pp. 4705–4721, Jan. 2016, doi: https://doi.org/10.1007/s00429-015-1179-4
J. M. Soares, P. Marques, V. Alves, N. Sousa, “A hitchhiker’s guide to diffusion tensor imaging,” Front. Neurosci., vol. 7, art. no. 31, Mar. 2013, doi: https://doi.org/10.3389/fnins.2013.00031
S.-K. Song, S.-W. Sun, M. J. Ramsbottom, C. Chang, J. Russell, A. H. Cross, “Dysmyelination Revealed through MRI as Increased Radial (but Unchanged Axial) Diffusion of Water,” Neuroimage, vol. 17, no. 3, pp. 1429–1436, Nov. 2002, doi: https://doi.org/10.1006/nimg.2002.1267
C. A. M. Wheeler-Kingshott, M. Cercignani, “About “axial” and “radial” diffusivities,” Magn. Reson. Med., vol. 61, no. 5, pp. 1255–1260, May 2009, doi: https://doi.org/10.1002/mrm.21965
M. Jenkinson, C. F. Beckmann, T. E. J. Behrens, M. W. Woolrich, S. M. Smith, “FSL,” Neuroimage, vol. 62, no. 2, pp. 782–790, Aug. 2012, doi: https://doi.org/10.1016/j.neuroimage.2011.09.015
Analysis Group, FDT/UserGuide. (2022). Accessed: Jun. 10, 2023. [Online]. Available: https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT/UserGuide#DTIFIT
T. Koyama, Y. Uchiyama, K. Domen, “Outcome in Stroke Patients Is Associated with Age and Fractional Anisotropy in the Cerebral Peduncles: A Multivariate Regression Study,” Prog. Rehabil. Med., vol. 5, art. no. 20200006, 2020, doi: https://doi.org/10.2490/prm.20200006
J. Chen, M. Liu, D. Sun, Y. Jin, T. Wang, C. Ren, “Effectiveness and neural mechanisms of home-based telerehabilitation in patients with stroke based on fMRI and DTI: A study protocol for a randomized controlled trial,” Medicine, vol. 97, no. 3, art. no. e9605, Jan. 2018, doi: https://doi.org/10.1097/MD.0000000000009605
S. Mori, K. Oishi, H. Jiang, L. Jiang, et al., “Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template,” Neuroimage, vol. 40, no. 2, pp. 570–582, Apr. 2008, doi: https://doi.org/10.1016/j.neuroimage.2007.12.035
J. C. Eliassen, E. L. Boespflug, M. Lamy, J. Allendorfer, W.-J. Chu, J. P. Szaflarski, “Brain-Mapping Techniques for Evaluating Poststroke Recovery and Rehabilitation: A Review,” Top. Stroke Rehabil., vol. 15, no. 5, pp. 427–450, 2008, doi: https://doi.org/10.1310/tsr1505-427
R. G. González, “Clinical MRI of acute ischemic stroke,” J. Magn. Reson. Imaging, vol. 36, no. 2, pp. 259–271, Aug. 2012, doi: https://doi.org/10.1002/jmri.23595
J. M. Wardlaw, W. Brindle, A. M. Casado, K. Shuler, et al., “A systematic review of the utility of 1.5 versus 3 Tesla magnetic resonance brain imaging in clinical practice and research,” Eur. Radiol., vol. 22, no. 11, pp. 2295–2303, Nov. 2012, doi: https://doi.org/10.1007/s00330-012-2500-8
E. Seto, G. Sela, W. E. Mcllroy, S. E. Black, et al., “Quantifying Head Motion Associated with Motor Tasks Used in fMRI,” Neuroimage, vol. 14, no. 2, pp. 284–297, Aug. 2001, doi: https://doi.org/10.1006/nimg.2001.0829
M. Veldsman, T. Cumming, A. Brodtmann, “Beyond BOLD: Optimizing functional imaging in stroke populations,” Hum. Brain Mapp., vol. 36, no. 4, pp. 1620–1636, Apr. 2015, doi: https://doi.org/10.1002/hbm.22711
Q. Guo, G. Hall, M. Mckinnon, R. Goeree, E. Pullenayegum, “Setting sample size using cost efficiency in fMRI studies,” Open Access Med. Stat., vol. 2, pp. 33–41, May 2012, doi: https://doi.org/10.2147/OAMS.S30830
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Revista Mexicana de Ingenieria Biomedica

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Upon acceptance of an article in the RMIB, corresponding authors will be asked to fulfill and sign the copyright and the journal publishing agreement, which will allow the RMIB authorization to publish this document in any media without limitations and without any cost. Authors may reuse parts of the paper in other documents and reproduce part or all of it for their personal use as long as a bibliographic reference is made to the RMIB. However written permission of the Publisher is required for resale or distribution outside the corresponding author institution and for all other derivative works, including compilations and translations.







