Novel Studies in the Designs of Natural, Synthetic, and Compound Hydrogels with Biomedical Applications
DOI:
https://doi.org/10.17488/RMIB.44.2.6Keywords:
drug delivery system, hidrogel, regenerative medicine, wound repairAbstract
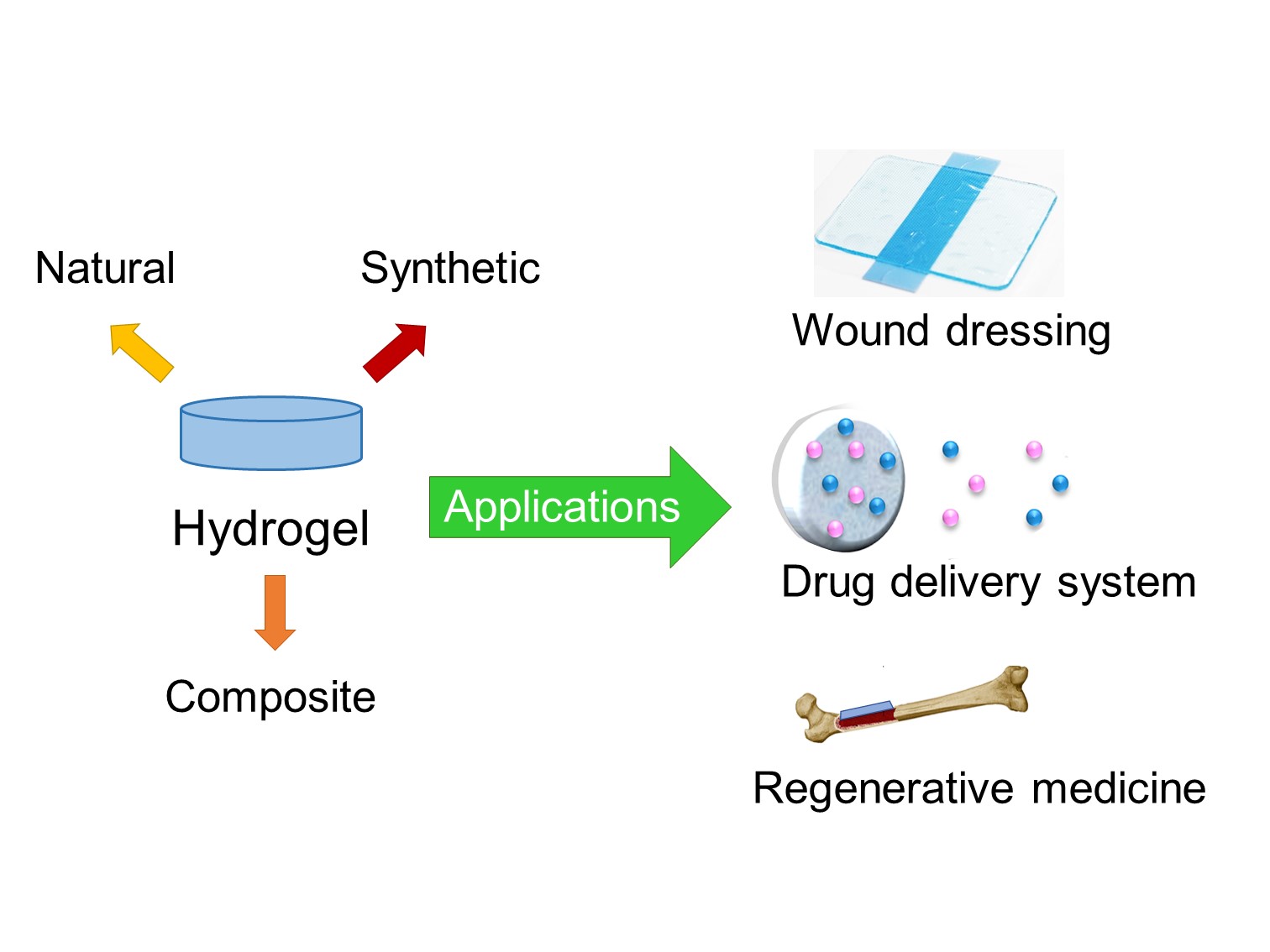
Hydrogels are gaining widespread popularity in the biomedical field due to their extraordinary properties, such as biocompatibility, biodegradability, zero toxicity, easy processing, and similarity to physiological tissue. They have applications in controlled drug release, wound dressing, tissue engineering, and regenerative medicine. Among these applications, hydrogels as a controlled drug delivery system stands out, which releases active substances in precise amounts and at specific times. To explore the latest advances in the design of hydrogels, a literature review of articles published in indexed scientific journals, in Scopus and Science Direct, was carried out. This review aimed to discover and describe the most innovative hydrogel research with applications in the biomedical field; hydrogels synthesized with polymers of different origins were selected, such as; i. Natural (dextran, agarose, chitosan, etc.); ii. Synthetic (polyacrylamide, polyethylene glycol, polyvinyl alcohol, etc.); iii. Composites (interpenetrants, hybrid crosslinkers, nanocomposites, etc.). Comparative analysis revealed that hydrogels with composite materials show the most promise. These composite hydrogels combine the advantages of different polymers or incorporate additional components, offering enhanced properties and functionalities. In summary, hydrogels are versatile biomaterials with immense potential in biomedicine. Their unique properties make them suitable for diverse applications. However, innovative designs and formulations must continue to be explored to further advance the capabilities of hydrogels and expand their biomedical applications.
Downloads
References
J. A. Cortés, J. E. Puig, J. A. Morales, E. Mendizábal, “Thermosensitive nanostructured hydrogels synthesized by inverse microemulsion polymerization,” Rev. Mex. Ing. Quim., vol. 10, no. 3, pp. 513-520, 2011. [Online]. Available: https://www.scielo.org.mx/pdf/rmiq/v10n3/v10n3a16.pdf
P. Gami, D. Kundu, S. D. Seera, T. Banerjee, “Chemically crosslinked xylan–β-Cyclodextrin hydrogel for the in vitro delivery of curcumin and 5-Fluorouracil,” Int. J. Biol. Macromol., vol. 158, pp. 18-31, Sep. 2020, doi: https://doi.org/10.1016/j.ijbiomac.2020.04.237
N. Guo, L. Zhang, J. Wang, S. Wang, Y. Zou, X. Wang, “Novel fabrication of morphology tailored nanostructures with Gelatin/Chitosan Co-polymeric bio-composited hydrogel system to accelerate bone fracture healing and hard tissue nursing care management,” Process. Biochem., vol. 90, pp. 177-183, Mar. 2020, doi: https://doi.org/10.1016/j.procbio.2019.11.016
M. Martínez-Martínez, G. Rodríguez-Berna, M. Bermejo, I. González-Alvarez, M. González-Alvarez, V. Merino, “Covalently crosslinked organophosphorus derivatives-chitosan hydrogel as a drug delivery system for oral administration of camptothecin,” Eur. J. Pharm. Biopharm., vol. 136, pp. 174-183, Mar. 2019, doi: https://doi.org/10.1016/j.ejpb.2019.01.009
J. Qu, Y. Liang, M. Shi, B. Guo, Y. Gao, Z. Yin, “Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release,” Int. J. Biol. Macromol., vol. 140, pp. 255-264, Nov. 2019, doi: https://doi.org/10.1016/j.ijbiomac.2019.08.120
S. Omidi, M. Pirhayati, A. Kakanejadifard, “Co-delivery of doxorubicin and curcumin by a pH-sensitive, injectable, and in situ hydrogels composed of chitosan, graphene, and cellulose nanowhisker,” Carbohydr. Polym., vol. 231, art. no. 115745, Mar. 2020, doi: https://doi.org/10.1016/j.carbpol.2019.115745
T. Takei, R. Yoshihara, S. Danjo, Y. Fukuhara, et al., “Hydrophobically-modified gelatin hydrogel as a carrier for charged hydrophilic drugs and hydrophobic drugs,” Int. J. Biol. Macromol., vol. 149, pp. 140-147, Apr. 2020, doi: https://doi.org/10.1016/j.ijbiomac.2020.01.227
C. A. Dreiss, “Hydrogel design strategies for drug delivery,” Curr. Opin. Colloid Interface Sci., vol. 48, pp. 1-17, Aug. 2020, doi: https://doi.org/10.1016/j.cocis.2020.02.001
M. S. Amini-Fazl, R. Mohammadi, K. Kheiri, “5‑Fluorouracil loaded chitosan/polyacrylic acid/Fe3O4 magnetic nanocomposite hydrogel as a potential anticancer drug delivery system,” Int. J. Biol. Macromol., vol. 132, pp. 506-513, Jul. 2019, doi: https://doi.org/10.1016/j.ijbiomac.2019.04.005
G. F. B. Almeida, M. R. Cardoso, D. C. Zancanela, L. L. Bernarde, et al., “Controlled drug delivery system by fs-laser micromachined biocompatible rubber latex membranes,” Appl. Surf. Sci., vol. 506, art. no. 144762, Mar. 2020, doi: https://doi.org/10.1016/j.apsusc.2019.144762
S. H. Aswathy, U. Narendrakumar, I. Manjubala, “Commercial hydrogels for biomedical applications,” Heliyon, vol. 6, no. 4, art. no. e03719, Apr. 2020, doi: https://doi.org/10.1016/j.heliyon.2020.e03719
M. Tenje, F. Cantoni, A. M. Porras-Hernández, S. S. Searle, et al., “A practical guide to microfabrication and patterning of hydrogels for biomimetic cell culture scaffolds,” Organs-on-a-Chip, art. no. 100003, Dec. 2020, doi: https://doi.org/10.1016/j.ooc.2020.100003
J. Xiang, L. Shen, Y. Hong, “Status and future scope of hydrogels in wound healing: Synthesis, materials, and evaluation,” Eur. Polym. J., vol. 130, art. no. 109609, May 2020, doi: https://doi.org/10.1016/j.eurpolymj.2020.109609
S. Ata, A. Rasool, A. Islam, I. Bibi, et al., “Loading of Cefixime to pH-sensitive chitosan-based hydrogel and investigation of controlled release kinetics,” Int. J. Biol. Macromol., pp. 1236-1244, Jul. 2020, doi: https://doi.org/10.1016/j.ijbiomac.2019.11.091
Y. Qiao, S. Xu, T. Zhu, N. Tang, X. Bai, C. Zheng, “Preparation of printable double-network hydrogels with rapid self-healing and high elasticity based on hyaluronic acid for controlled drug release,” Polymer, art. no. 121994, Jan. 2020, doi: https://doi.org/10.1016/j.polymer.2019.121994
L. de Y. Pozzo, T. F. da Conceição, A. Spinelli, N. Scharnagl, “Chitosan coatings crosslinked with genipin for corrosion protection of AZ31 magnesium alloy sheets,” Carbohydr. Polym., vol. 181, pp. 71-77, Feb. 2018, doi: https://doi.org/10.1016/j.carbpol.2017.10.055
H. Samadian, H. Maleki, A. Fathollahi, M. Salehi, et al., “Naturally occurring biological macromolecules-based hydrogels: Potential biomaterials for peripheral nerve regeneration,” Int. J. Biol. Macromol., vol. 154, pp. 795-817, Jul. 2020, doi: https://doi.org/10.1016/j.ijbiomac.2020.03.155
M. Farrag, S, Abri, N. Leipzig, “pH-dependent RNA isolation from cells encapsulated in chitosan-based biomaterials,” Int. J. Biol. Macromol., vol. 146, pp. 422-430, Mar. 2020, doi: https://doi.org/10.1016/j.ijbiomac.2019.12.263
N. A. O'Connor, M. Jitianu, G. Nunez, Q. Picard, et al., “Dextran hydrogels by crosslinking with amino acid diamines and their viscoelastic properties,” Int. J. Biol. Macromol., vol. 111, pp. 370-378, May 2018, doi: https://doi.org/10.1016/j.ijbiomac.2018.01.042
M. C. Stanciu, M. Nichifor, “Influence of dextran hydrogel characteristics on adsorption capacity for anionic dyes,” Carbohydr. Polym., vol. 199, pp. 75-83, Nov. 2018, doi: https://doi.org/10.1016/j.carbpol.2018.07.011
J. E. Lee, W. H. Seung, H. K. Chae, J. P. Seong, P. Suk-Hee, K. Tae Hee, “In-situ ionic crosslinking of 3D bioprinted cell-hydrogel constructs for mechanical reinforcement and improved cell growth,” Biomater. Adv., vol. 147, art. no. 213322, Apr. 2023, doi: https://doi.org/10.1016/j.bioadv.2023.213322
D. A. Gyles, L. D. Castro, J. O. Carréra Silva, R. M. Ribeiro-Costa, “A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations,” Eur. Polym. J., vol. 88, pp. 373-392, Mar. 2017, doi: https://doi.org/10.1016/j.eurpolymj.2017.01.027
L. Li, F. Yu, L. Zheng, R. Wang, et al., “Natural hydrogels for cartilage regeneration: Modification, preparation, and application,” J. Orthop. Trans., vol. 17, pp. 26-41, Apr. 2019, doi: https://doi.org/10.1016/j.jot.2018.09.003
Z, Liu, Z. Tang, L. Zhu, S. Lu, et al., “Natural protein-based hydrogels with high strength and rapid self-recovery,” Int. J. Biol. Macromol., vol. 141, pp. 108-116, Dec. 2019, doi: https://doi.org/10.1016/j.ijbiomac.2019.08.258
P. Nikpour, H. Salimi-Kenari, F. Fahimipour, S. M. Rabbie, M. Imani, E. Dashtimoghadam, L. Tayebi, “Dextran hydrogels incorporated with bioactive glass-ceramic: Nanocomposite scaffolds for bone tissue engineering,” Carbohydr. Polym., vol. 190, pp. 281-294, Jun. 2018, doi: https://doi.org/10.1016/j.carbpol.2018.02.083
M. Zhang, Y. Huang, W. Pan, X. Tong, et al., “Polydopamine-incorporated dextran hydrogel drug carrier with the tailorable structure for wound healing,” Carbohydr. Polym., vol. 253, art. no. 117213, Feb. 2021, doi: https://doi.org/10.1016/j.carbpol.2020.117213
C. Zheng, C. Liu, H. Chen, N. Wang, X. Liu, G. Sun, W. Qiao, “Effective wound dressing based on Poly (vinyl alcohol)/Dextran-aldehyde composite hydrogel,” Int. J. Biol. Macromol., vol. 132, pp. 1098-1105, Jul. 2019, doi: https://doi.org/10.1016/j.ijbiomac.2019.04.038
R. Ghaffari, H. Salimi-Kenari, F. Fahimipour, S. M. Rabbie, H. Adeli, E. Dashtimoghadam, “Fabrication and characterization of dextran/nanocrystalline β-tricalcium phosphate nanocomposite hydrogel scaffolds,” Int. J. Biol. Macromol., vol. 148, pp. 434-448, Apr. 2020, doi: https://doi.org/10.1016/j.ijbiomac.2020.01.112
S. O. Solomevich, P. M. Bychkovsky, T. L. Yurkshtovich, N. V. Golub, P. Y. Mirchuk, M. Y. Revtovich, A. I. Shmak, “Biodegradable pH-sensitive prospidine-loaded dextran phosphate-based hydrogels for local tumor therapy,” Carbohydr. Polym., vol. 226, art. no. 115308, Dec. 2019, doi: https://doi.org/10.1016/j.carbpol.2019.115308
P. Nonsuwan, A. Matsugami, F. Hayashi, S.-H. Hyon, K. Matsumura, “Controlling the degradation of an oxidized dextran-based hydrogel independent of the mechanical properties,” Carbohydr. Polym., vol. 204, pp. 131-141, Jan. 2019, doi: https://doi.org/10.1016/j.carbpol.2018.09.081
D.-S. Kang, S.-Y. Yang, C.-Y. Lee, “Fabrication of innocuous hydrogel scaffolds based on modified dextran for biotissues,” Carbohydr. Res., vol. 522, art. no. 108699, Dec. 2022, doi: https://doi.org/10.1016/j.carres.2022.108699
J. Shen, W. Jiao, Z. Chen, C. Wang, et al., “Injectable multifunctional chitosan/dextran-based hydrogel accelerates wound healing in combined radiation and burn injury,” Carbohydr. Polym., vol. 316, art. no. 121024, Sep. 2023, doi: https://doi.org/10.1016/j.carbpol.2023.121024
M. D. L. L. R. Menezes, H. L. Ribeiro, F. O. M. D. S. Abreu, J. P. A. Feitosa, M. S. M. S. Filho, “Optimization of the collagen extraction from Nile tilapia skin (Oreochromis niloticus) and its hydrogel with hyaluronic acid,” Colloids Surf. B, vol. 189, art. no. 110852, May 2020, doi: https://doi.org/10.1016/j.colsurfb.2020.110852
Y. Fang, L. Shi, Z. Duan, S. Rohani, “Hyaluronic acid hydrogels, as a biological macromolecule-based platform for stem cells delivery and their fate control: A review,” Int. J. Biol. Macromol., vol. 189, pp. 554-566, Oct. 2021, doi: https://doi.org/10.1016/j.ijbiomac.2021.08.140
J. Luo, Z. Wu, Y. Lu, K. Xiong, et al., “Intraperitoneal administration of biocompatible hyaluronic acid hydrogel containing multi-chemotherapeutic agents for the treatment of colorectal peritoneal carcinomatosis,” Int. J. Biol. Macromol., vol. 152, pp. 718-726, Jun. 2020, doi: https://doi.org/10.1016/j.ijbiomac.2020.02.326
W. Wang, D. Shi, Y. Zhang, W. Li, et al., “An injectable hydrogel based on hyaluronic acid prepared by Schiff base for long-term controlled drug releasee,” Int. J. Biol. Macromol., vol. 245, art. no. 125341, Aug. 2023, doi: https://doi.org/10.1016/j.ijbiomac.2023.125341
N.-G. Kim, P. Chandika, S.-C. Kim, D.-H. Won, et al., “Fabrication and characterization of ferric ion cross-linked hyaluronic acid/pectin-based injectable hydrogel with antibacterial ability,” Polymer, vol. 271, art. no. 125808, Apr. 2023, doi: https://doi.org/10.1016/j.polymer.2023.125808
S.R Batool, M. A. Nazeer, D. Ekinci, A. Sahin, S. Kizilel, “Multifunctional alginate-based hydrogel with reversible crosslinking for controlled therapeutics delivery,” Int. J. Biol. Macromol., vol. 150, pp. 315-325, May 2020, doi: https://doi.org/10.1016/j.ijbiomac.2020.02.042
B. M. Millán-Olvera, B. García-Gaitán, I. Ruiz-Aguilar, M. Flores-Castañeda, N. Ríos-Donato, J. L. García-Rivas, “Obtención y caracterización de perlas de Alginato-imidacloprid y alginato-bifentrina,” Afinidad, vol. 77, art. no. 590, 2020. [Online]. Available: https://raco.cat/index.php/afinidad/article/view/371257
A. R. Abbasi, M. Sohail, M. U. Minhas, T. Khaliq, M. Kousar, S. Khan, Z. Hussain, A. Munir, “Bioinspired sodium alginate-based thermosensitive hydrogel membranes for accelerated wound healing,” Int. J. Biol. Macromol., vol. 155, pp. 751-765, Jul. 2020 doi: https://doi.org/10.1016/j.ijbiomac.2020.03.248
A. Remes, D. Basha, T. Puehler, C. Borowski, et al., “Alginate hydrogel polymers enable efficient delivery of a vascular-targeted AAV vector into aortic tissue,” Mol. Ther. Methods Clin. Dev., vol. 21, pp. 83-93, Jun. 2021, doi: https://doi.org/10.1016/j.omtm.2021.02.017
R. Zhang, L. Lei, Q. Song, X. Li, “Calcium ion cross-linking alginate/dexamethasone sodium phosphate hybrid hydrogel for extended drug release,” Colloids Surf. B, vol. 175, pp. 569-575, Mar. 2019, doi: https://doi.org/10.1016/j.colsurfb.2018.11.083
X. Sun, C. Ma, W. Gong, Y. Ma, Y. Ding, L. Liu, “Biological properties of sulfanilamide-loaded alginate hydrogel fibers based on ionic and chemical crosslinking for wound dressings,” Int. J. Biol. Macromol., vol. 157, pp. 522-529, Aug. 2020, doi: https://doi.org/10.1016/j.ijbiomac.2020.04.210
X. Yang, W. Mo, Y. Shi, X. Fang, Y. Xu, X. He, Y. Xu, “Fumaria officinalis-loaded chitosan nanoparticles dispersed in an alginate hydrogel promote diabetic wounds healing by upregulating VEGF, TGF-β, and b-FGF genes: A preclinical investigation,” Helyon, vol. 9, no. 7, art. no. e17704, Jul. 2023, doi: https://doi.org/10.1016/j.heliyon.2023.e17704
G.-Q. Fu, S.-C. Zhang, G.-G. Chen, X. Hao, J. Bian, F. Peng, “Xylan-based hydrogels for potential skincare application,” Int. J. Biol. Macromol., vol. 158, pp. 244-250, Sep. 2020, doi: https://doi.org/10.1016/j.ijbiomac.2020.04.235
S. Sharifi, M. M. Islam, H. Sharifi, R. Islam, et al., “Tuning gelatin-based hydrogel towards bioadhesive ocular tissue engineering applications,” Bioact. Mater., vol. 6, no. 11, pp. 3947-3961, Nov. 2021, doi: https://doi.org/10.1016/j.bioactmat.2021.03.042
T. Takei, R. Yoshihara, S. Danjo, Y. Fukuhara, et al., “Hydrophobically-modified gelatin hydrogel as a carrier for charged hydrophilic drugs and hydrophobic drugs,” Int. J. Biol. Macromol., vol. 149, pp. 140-147, Apr. 2020, doi: https://doi.org/10.1016/j.ijbiomac.2020.01.227
M. Anamizu, Y. Tabata, “Design of injectable hydrogels of gelatin and alginate with ferric ions for cell transplantation,” Acta Biomater., vol. 100, pp. 184-190, Dec. 2019, doi: https://doi.org/10.1016/j.actbio.2019.10.001
G. González-Ulloa, M. Jiménez-Rosado, M. Rafii-El-Idrissi Benhnia, A. Romero, E. Ruiz-Mateos, F.J. Ostos, V. Perez-Puyana, “Hybrid polymeric Hydrogel-based biomaterials with potential applications in regenerative medicine,” J. Mol. Liq., vol. 384, art. no. 122224, Aug. 2023, doi: https://doi.org/10.1016/j.molliq.2023.122224
A. Alehosseini, E.-M. Gomez del Pulgar, M. J. Fabra, L. G. Gómez-Mascaraque, et al., “Agarose-based freeze-dried capsules prepared by the oil-induced biphasic hydrogel particle formation approach for the protection of sensitive probiotic bacteria,” Food Hydrocoll., vol. 87, pp. 487-496, Feb. 2019, doi: https://doi.org/10.1016/j.foodhyd.2018.08.032
B. Bagheri, P. Zarrintaj, S. S. Surwase, N. Baheiraei, et al., “Self-gelling electroactive hydrogels based on chitosan–aniline oligomers/agarose for neural tissue engineering with on-demand drug release,” Colloids Surf. B, vol. 184, art. no. 110549, Dec. 2019, doi: https://doi.org/10.1016/j.colsurfb.2019.110549
Y. Yuan, L. Wang, R.-J. Mu, J. Gong, et al., “Effects of konjac glucomannan on the structure, properties, and drug release characteristics of agarose hydrogels,” Carbohydr. Polym., vol. 190, pp. 196-203, Jun. 2018, doi: https://doi.org/10.1016/j.carbpol.2018.02.049
J. Li, C. Wu, P. K. Chu, M. Gelinsky, “3D printing of hydrogels: Rational design strategies and emerging biomedical applications,” Mater. Sci. Eng. R. Rep., vol. 140, art. no. 100543, Apr. 2020, doi: https://doi.org/10.1016/j.mser.2020.100543
F. Topuz, A. Nadernezhad, O. S. Caliskan, Y. Z. Menceloglu, B. Kac, “Nanosilicate embedded agarose hydrogels with improved bioactivity,” Carbohydr. Polym., vol. 201, pp. 105-112, Dec. 2018, doi: https://doi.org/10.1016/j.carbpol.2018.08.032
X. Qi, T. Su, X. Tong, W. Xiong, et al., “Facile formation of sale can/agarose hydrogels with tunable structural properties for cell culture,” Carbohydr. Polym., vol. 224, art. no. 115208, Nov. 2019, doi: https://doi.org/10.1016/j.carbpol.2019.115208
M.I. Patiño Vargas, F.D. Martinez-Garcia, F. Offens, N.Y. Becerra, et al., Viscoelastic properties of plasma-agarose hydrogels dictate favorable fibroblast responses for skin tissue engineering applications,” Biomater. Adv., vol. 139, art. no. 212967, Aug. 2022, doi: https://doi.org/10.1016/j.bioadv.2022.212967
R. García-González, R. E. Zavala-Arce, P. Ávila-Pérez, B. García-Gaitán, J. L. González-Chávez, C. Muro-Urista, G. Luna-Bárcenas, “Síntesis y caracterización de un material criogénico a partir de quitosano y celulosa,” Afinidad, vol. 71, art. no. 567, 2014. [Online]. Available: https://raco.cat/index.php/afinidad/article/view/281148/368860
J. O. Gonçalves, J. P. Santos, E. C. Rios, M. M. Crispim, G. L. Dotto, L. A. A. Pinto, “Development of chitosan-based hybrid hydrogels for dyes removal from an aqueous binary system,” J. Mol. Liq., vol. 225, pp. 265-270, Jun. 2019, doi: https://doi.org/10.1016/j.molliq.2016.11.067
K. Kaur, R. Jindal, “Comparative study on the behavior of Chitosan-Gelatin based Hydrogel and nanocomposite ion exchanger synthesized under microwave conditions towards photocatalytic removal of cationic dyes,” Carbohydr. Polym., vol. 207, pp. 398-410, Mar. 2019, doi: https://doi.org/10.1016/j.carbpol.2018.12.002
M. Imran, M. Sajwan, B. Alsuwayt, M. Asif, “Synthesis, characterization, and anticoagulant activity of chitosan derivatives,” Saudi Pharm. J., vol. 28, no. 1, pp. 25-32, Jan. 2020, doi: https://doi.org/10.1016/j.jsps.2019.11.003
P. S. Pauletto, J. O. Gonçalves, L. A. A. Pinto, G. L. Dotto, N. P. G. Salau, “Single and competitive dye adsorption onto chitosan-based hybrid hydrogels using artificial neural network modeling,” J. Colloid Interface Sci., vol. 560, pp. 722-729, Feb. 2020, doi: https://doi.org/10.1016/j.jcis.2019.10.106
L. Quihui-Cota, G. G. Morales-Figueroa, E. Valbuena-Gregorio, J. C. Campos-García, N. P. Silva-Beltrán, M. A. López-Mata, “Membrana de Quitosano con Aceites Esenciales de Romero y Árbol de Té: Potencial como Biomaterial,” Rev. Mex. Ing. Biomed., vol. 38, no. 1, pp. 255 -264, Jan. 2017, doi: https://doi.org/10.17488/RMIB.38.1.20
A. M. Heimbuck, T. R. Priddy-Arrington, B. J. Sawyer, M. E. Caldorera-Moore, “Effects of post-processing methods on chitosan-genipin hydrogel properties,” Mater. Sci. Eng. C, vol. 98, pp. 612-618, May 2019, doi: https://doi.org/10.1016/j.msec.2018.12.119
H. Tashakkorian, V. Hasantabar, A. Mostafazadeh, M. Golpour, “Transparent chitosan-based nanocomposite hydrogel: Synthesis, thermophysical characterization, cell adhesion, and viability assay,” Int. J. Biol. Macromol., vol. 144, pp. 715-724, Feb. 2020, doi: https://doi.org/10.1016/j.ijbiomac.2019.10.157
T. Songkroh, H. Xie, W. Yu, G. Lv, et al., “Erratum to: In situ forming chitosan-based hydrogel as a lung sealant for biological lung volume reduction,” Sci. Bull., vol. 60, no. 2, pp. 235-240, Jan. 2015, doi: https://doi.org/10.1007/S11434-014-0548-3
D. Dehghan-Baniani, Y. Chen, D. Wang, R. Bagheri, A. Solouk, H. Wu, “Injectable in situ forming kartogenin-loaded chitosan hydrogel with tunable rheological properties for cartilage tissue engineering,” Colloids Surf. B, vol. 192, art. no. 111059, Aug. 2020, doi: https://doi.org/10.1016/j.colsurfb.2020.111059
K. Thongchai, P. Chuysinuan, T. Thanyacharoen, S. Techasakul, S. Ummartyotin, “Characterization, release, and antioxidant activity of caffeic acid-loaded collagen and chitosan hydrogel composites,” J. Mater. Res. Technol., vol. 9, no. 3, pp. 6512-6520, May 2020, doi: https://doi.org/10.1016/j.jmrt.2020.04.036
J. Wang, W. Xu, W. Zhang, J. Da, et al., “UV cross-linked injectable non-swelling dihydrocaffeic acid grafted chitosan hydrogel for promoting wound healing,” Carbohydr. Polym., vol. 314, art. no. 120926, Aug. 2023, doi: https://doi.org/10.1016/j.carbpol.2023.120926
J. M. Gutiérrez-Hernández, C. Castorena-Alejandro, D. M. Escobar-García, A. Escalante, et al., “In vitro evaluation of spruce xylan/MWCNTs hydrogel scaffolds for bone regeneration,” Mater. Today. Commun., vol. 35, art. no. 106070, Jun. 2023, doi: https://doi.org/10.1016/j.mtcomm.2023.106070
Y. Kambe, “Functionalization of silk fibroin-based biomaterials for tissue engineering,” Polym. J., vol. 53, Jul. 2021, doi: https://doi.org/10.1038/s41428-021-00536-5
Z. Li, J. Song, J. Zhang, K. Hao, et al., “Topical application of silk fibroin-based hydrogel in preventing hypertrophic scars,” Colloids Surf. B, vol. 186, art. no. 110735, Feb. 2020, doi: https://doi.org/10.1016/j.colsurfb.2019.110735
D. Gaviria Arias, L. C. Caballero Mendez, “Fibroin from silkworm (Bombix mori L.) as biomaterial used in regenerative medicine process based on tissue engineering,” Rev. Méd. Risaralda, vol. 21, no. 1, pp. 38-47, 2015. [Online]. Available: http://www.scielo.org.co/pdf/rmri/v21n1/v21n1a08.pdf
H. Wang, H. Wan, Q. Wang, Y. Ma, G. Su, X. Cao, H. Gao, “Engineered multifunctional silk fibroin/gelatin hydrogel conduit loaded with miR-29a@ZIF-8 nanoparticles for peripheral nerve regeneration,” Smart Mater. Med., vol. 4, pp. 480-496, 2023, doi: https://doi.org/10.1016/j.smaim.2023.02.002
L. D. Amer, L. S. Saleh, C. Walker, S. Thomas, W. J. Janssen, S. Alper, S. J. Bryant, “Inflammation via myeloid differentiation primary response gene 88 signaling mediates the fibrotic response to implantable synthetic poly(ethylene glycol) hydrogels,” Acta Biomater., vol. 100, pp. 105-117, Dec. 2019, doi: https://doi.org/10.1016/j.actbio.2019.09.043
M. Guvendiren, J. A. Burdick, “Engineering synthetic hydrogel microenvironments to instruct stem cells,” Curr. Opin. Biotechnol., vol. 24, no. 5, pp. 841-846, Oct. 2013, doi: https://doi.org/10.1016/j.copbio.2013.03.009
R. Cruz-Acuña, A. J. García, “Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions,” Matrix Biol., vol. 57-58, pp. 324-333, Jan. 2017, doi: https://doi.org/10.1016/j.matbio.2016.06.002
N. S. Alghunaim, “Characterization of selenium oxide nanofiller effect on the spectroscopic and thermal properties of Cs/PAM nanocomposites,” J. Mater. Res. Technol., vol. 9, no. 3, pp. 3502-3510, 2020, doi: https://doi.org/10.1016/j.jmrt.2020.01.087
D. Zhao, M. Feng, L. Zhang, B. He, X. Chen, J. Sun, “Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond,” Carbohydr. Polym., vol. 256, art. no. 117580, Mar. 2021, doi: https://doi.org/10.1016/j.carbpol.2020.117580
Y. Zhang, H. Chen, Y. Li, A. Fang, et al., “A transparent sericin-polyacrylamide interpenetrating network hydrogel as visualized dressing material,” Polym. Test., vol. 87, art. no. 106517, Jul. 2020, doi: https://doi.org/10.1016/j.polymertesting.2020.106517
S. R. McClure, C. Wang, “A Preliminary Field Trial Evaluating the Efficacy of 4% Polyacrylamide Hydrogel in Horses With Osteoarthritis,” J. Equine Vet. Sci., vol. 54, pp. 98-102, Jul. 2017, doi: https://doi.org/10.1016/j.jevs.2017.02.019
Y. Chen, X. Fan, X. Liu, C. Meng, et al., “Highly stretchable, adhesive and antibacterial double-network hydrogels toward flexible strain sensor,” Polym. Test., vol. 124, art. no. 108087, Jul. 2023, doi: https://doi.org/10.1016/j.polymertesting.2023.108087
Z. Liu, W. Tang, J. Liu, Y. Han, et al., “A novel sprayable thermosensitive hydrogel coupled with zinc modified metformin promotes the healing of skin wound,” Bioact. Mater., vol. 20, pp. 610-626, Feb. 2023, doi: https://doi.org/10.1016/j.bioactmat.2022.06.008
A. R. Kim, S. L. Lee, S. N. Park, “Properties and in vitro drug release of pH- and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropyl acrylamide) for transdermal delivery of luteolin,” Int. J. Biol. Macromol., vol. 118, pp. 731-740, Oct. 2018 doi: https://doi.org/10.1016/j.ijbiomac.2018.06.061
M. Martinez-Moro, J. Jenczyk, J. M. Giussi, S. Jurga, S. E. Moya, “Kinetics of the thermal response of poly(N-isopropylacrylamide co methacrylic acid) hydrogel microparticles under different environmental stimuli: A time-lapse NMR study,” J. Colloid Interface Sci., vol. 580, pp. 439-448, Nov. 2020, doi: https://doi.org/10.1016/j.jcis.2020.07.049
N. Shivshetty, T. Swift, A. Pinnock, D. Pownall, et al., “Evaluation of ligand modified poly (N-Isopropyl acrylamide) hydrogel for etiological diagnosis of corneal infection,” Exp. Eye Res., vol. 214, art. no. 108881, Jan. 2022, doi: https://doi.org/10.1016/j.exer.2021.108881
G. Damonte, M. Cozzani, D. Di Lisa, L. Pastorino, A. Mariani, O. Monticelli, “Mechanically-reinforced biocompatible hydrogels based on poly(N-isopropylacrylamide) and star-shaped polycaprolactones,” Eur. Polym. J., vol. 195, art. no. 112239, Aug. 2023, doi: https://doi.org/10.1016/j.eurpolymj.2023.112239
A. Janse van Rensburg, N. H. Davies, A. Oosthuysen, C. Chokoza, P. Zilla, D. Bezuidenhout, “Improved vascularization of porous scaffolds through growth factor delivery from heparinized polyethylene glycol hydrogels,” Acta Biomater., vol. 49, pp. 89-100, Feb. 2017, doi: https://doi.org/10.1016/j.actbio.2016.11.036
A. Navaratnam, J. Cumsky, H. Abdul-Mushin, J. Gagneur, et al., “Assessment of Polyethylene Glycol Hydrogel Spacer and Its Effect on Rectal Radiation Dose in Prostate Cancer Patients Receiving Proton Beam Radiation Therapy,” Adv. Radiat. Oncol., vol. 5, no. 1, pp. 92-100, Sep. 2019, doi: https://doi.org/10.1016/j.adro.2019.08.007
Y. Fan, M. Lüchow, A. Badria, D. J. Hutchinson, M. Malkoch, “Placenta Powder-Infused Thiol-Ene PEG Hydrogels as Potential Tissue Engineering Scaffolds,” Biomacromolecules, vol. 24, no. 4, pp. 1617-1626, 2023, doi: https://doi.org/10.1021/acs.biomac.2c01355
T.-M. De Witte, A. M. Wagner, L. E. Fratila-Apachitei, A. A. Zadpoor, N. A. Peppas, “Degradable Poly(Methyl Methacrylate)-co-Methacrylic Acid Nanoparticles for Controlled Delivery of Growth Factors for Bone Regeneration,” Tissue Eng. Part A, vol. 26, no. 23-24. pp. 1226-1242, Dec. 2020, doi: https://doi.org/10.1089/ten.tea.2020.0010
G. Jiménez, S. Venkateswaran, E. López-Ruiz, M. Perán, et al., “A soft 3D polyacrylate hydrogel recapitulates the cartilage niche and allows growth-factor free tissue engineering of human articular cartilage,” Acta Biomater., vol. 90, pp. 146-156, May 2019, doi: https://doi.org/10.1016/j.actbio.2019.03.040
A. Stepulane, K. Ahlgren, A. Rodriguez-Palomo, A. K. Rajasekharan, M. Andersson, “Lyotropic liquid crystal elastomers for drug delivery,” Colloids Surf. B, vol. 226, art. no. 113304, Jun. 2023, doi: https://doi.org/10.1016/j.colsurfb.2023.113304
Z. Bao, Z. Gu, J. Xu, M. Zhao, G. Liu, J. Wu, “Acid-responsive composite hydrogel platform with space-controllable stiffness and calcium supply for enhanced bone regeneration,” Chem. Eng. J., vol. 396, art. no. 125353, Sep. 2020, doi: https://doi.org/10.1016/j.cej.2020.125353
M. Li, R. Wei, C. Liu, H. Fang, et al., “A “T.E.S.T.” hydrogel bioadhesive assisted by corneal cross-linking for in situ sutureless corneal repair,” Bioact. Mater., vol. 25, pp. 333-346, Jul. 2023, doi: https://doi.org/10.1016/j.bioactmat.2023.02.006
A. S. Montaser, M. Rehan, M. E. El-Naggar, “pH-Thermosensitive hydrogel based on polyvinyl alcohol/sodium alginate/N-isopropyl acrylamide composite for treating re-infected wounds,” Int. J. Biol. Macromol., vol. 124, pp. 1016-1024, Mar. 2019, doi: https://doi.org/10.1016/j.ijbiomac.2018.11.252
N. Chunshom, P. Chuysinuan, S. Techasakul, S. Ummartyotin, “Dried-state bacterial cellulose (Acetobacter xylinum) and polyvinyl-alcohol-based hydrogel: An approach to a personal care material,” J. Sci. Adv. Mater. Dev., vol. 3, no. 3, pp. 296-302, Sep. 2018, doi: https://doi.org/10.1016/j.jsamd.2018.06.004
A. A. Shefa, T. Sultana, M. K. Park, S. Y. Lee, J.-G. Gwon, B.-T. Lee, “Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing,” Mater. Des., vol. 186, art. no. 108313, Jan. 2020, doi: https://doi.org/10.1016/j.matdes.2019.108313
Z. Huang, X. Xiao, X. Jiang, S. Yang, et al., “Preparation and evaluation of a temperature-responsive methylcellulose/polyvinyl alcohol hydrogel for stem cell encapsulation,” vol. 119, art. no. 107936, Feb. 2023, doi: https://doi.org/10.1016/j.polymertesting.2023.107936
P. Sánchez-Cid, A. Romero, M.J. Díaz, M.V. de-Paz, V. Perez-Puyana, “Chitosan-based hydrogels obtained via photoinitiated click polymer IPN reaction,” J. Mol. Liq., vol. 379, art. no. 121735, Jun. 2023, doi: https://doi.org/10.1016/j.molliq.2023.121735
H. Xue, L. Hu, Y. Xiong, X. Zhu, et al., “Quaternized chitosan-Matrigel-polyacrylamide hydrogels as a wound dressing for wound repair and regeneration,” Carbohydr. Polym., vol. 226, art. no. 115302, Dec. 2019, doi: https://doi.org/10.1016/j.carbpol.2019.115302
Q. Zhang, X. Liu, X. Ren, L. Duan, G. Gao, “Adenine-mediated adhesive and tough hydrogel based on hybrid crosslinking,” Eur. Polym. J., vol. 106, pp. 139-147, Sep. 2018, doi: https://doi.org/10.1016/j.eurpolymj.2018.07.018
W. Gong, R. Wang, H. Huang, Y. Hou, et al., “Construction of double network hydrogels using agarose and gallic acid with antibacterial and anti-inflammatory properties for wound healing,” Int. J. Biol. Macromol., vol. 227, pp. 698-710, Feb. 2023, doi: https://doi.org/10.1016/j.ijbiomac.2022.12.085
S. P. Pasaribu, M. Ginting, I. Masmur, J. Kaban, Hestina, “Silver chloride nanoparticles embedded in self-healing hydrogels with biocompatible and antibacterial properties,” J. Mol. Liq., vol. 310, art. no. 113263, Jul. 2020, doi: https://doi.org/10.1016/j.molliq.2020.113263
K. B. Narayanan, S. M. Choi, S. S. Han, “Biofabrication of Lysinibacillus sphaericus-reduced graphene oxide in three-dimensional polyacrylamide/carbon nanocomposite hydrogels for skin tissue engineering,” Colloids Surf. B, vol. 181, pp. 539-548, Sep. 2019, doi: https://doi.org/10.1016/j.colsurfb.2019.06.007
A. Kumar, H. Kaur, “Sprayed in-situ synthesis of polyvinyl alcohol/chitosan loaded silver nanocomposite hydrogel for improved antibacterial effects,” Int. J. Biol. Macromol., vol. 145, pp. 950-964, Feb. 2020, doi: https://doi.org/10.1016/j.ijbiomac.2019.09.186
X. Chen, M. Zhang, D. Zhu, J. Zhang, et al., “Photocrosslinkable carboxylated polyvinyl alcohol nanocomposite hydrogels with enhanced compressive strength and cell adhesion,” Eur. Polym. J., vol. 196, art. no. 112252, Sep. 2023, doi: https://doi.org/10.1016/j.eurpolymj.2023.112252
Y. Q. Almajidi, S. S. Abdullaev, B. G. Alani, E. A. M. Saleh, et al., “Chitosan-gelatin hydrogel incorporating polyvinyl alcohol and MnFe double-layered hydroxide nanocomposites with biological activity,” Int. J. Biol. Macromol., vol. 246, art. no. 125566, Aug. 2023, doi: https://doi.org/10.1016/j.ijbiomac.2023.125566
M. Imaizumi, R. Nakamura, Y. Nakaegawa, B. T. Dirja, et al., “Regenerative potential of basic fibroblast growth factor contained in biodegradable gelatin hydrogel microspheres applied following vocal fold injury: Early effect on tissue repair in a rabbit model,” Braz. J. Otorhinolaryngol., vol. 87, no. 3, pp. 274-282, May 2021, doi: https://doi.org/10.1016/j.bjorl.2019.09.003000
M. K. Xiang Ping, H. W. Zhi, N.S. Aziz, N. A. Hadri, N. F. Ghazalli, N. Yusop, “Optimization of agarose–alginate hydrogel bead components for encapsulation and transportation of stem cells,” J. Taibah Univ. Medical Sci., vol. 18, no. 1, pp. 104-116, Feb. 2023, doi: https://doi.org/10.1016/j.jtumed.2022.08.009
I. Hamouda, C. Labay, M. P. Ginebra, E. Nicol, C. Canal, “Investigating the atmospheric pressure plasma jet modification of a photo-cross-linkable hydrogel,” Polymer, vol. 192, art. no. 122308, Mar. 2020, doi: https://doi.org/10.1016/j.polymer.2020.122308
P. Cyganowski, D. Jermakowicz-Bartkowiak, P. Jamroz, P. Pohl, A. Dzimitrowicz, “Hydrogel-based nanocomposite catalyst containing uncoated gold nanoparticles synthesized using cold atmospheric pressure plasma for the catalytic decomposition of 4-nitrophenol,” Colloids Surf. A, vol. 582, art. no. 123886, Dec. 2019, doi: https://doi.org/10.1016/j.colsurfa.2019.123886
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Revista Mexicana de Ingenieria Biomedica

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Upon acceptance of an article in the RMIB, corresponding authors will be asked to fulfill and sign the copyright and the journal publishing agreement, which will allow the RMIB authorization to publish this document in any media without limitations and without any cost. Authors may reuse parts of the paper in other documents and reproduce part or all of it for their personal use as long as a bibliographic reference is made to the RMIB. However written permission of the Publisher is required for resale or distribution outside the corresponding author institution and for all other derivative works, including compilations and translations.







